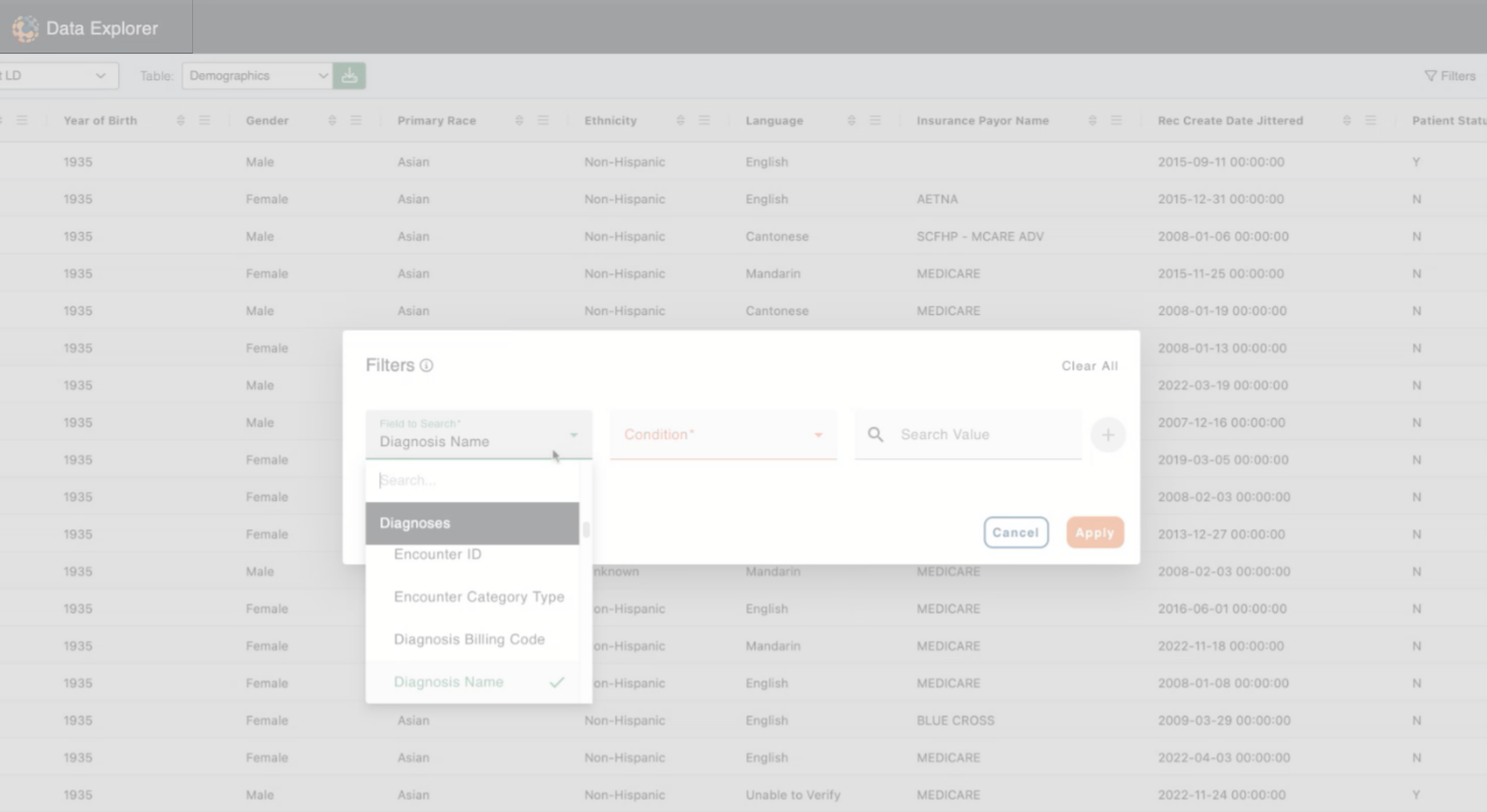
Hepatology Data
Leading real-world registry of liver disease patients covering chronic conditions across the spectrum of etiologies
Powering Hepatology Research
Target RWE offers the world's most comprehensive real-world evidence (RWE) data in hepatology, encompassing over 600,000 patients across U.S. hospitals, clinics, and physician-owned practices. With more than 15 million months of longitudinal follow-up, this extensive data provides unparalleled insights into liver disease progression, treatment efficacy, and patient outcomes.
Complete Patient Journeys Creating a Fit-For-Purpose Dataset
Target RWE's hepatology data captures a comprehensive range of patient information including demographics, vital signs, medical history, procedures, imaging, and lab results to drive clinical research and understanding of real-world patients suffering from chronic liver disease. Target RWE curates and transforms clinically derived health data making it available for research to enhance the understanding of disease mechanisms and progression, diverse patient subgroups, clinical outcomes and treatment effectiveness.
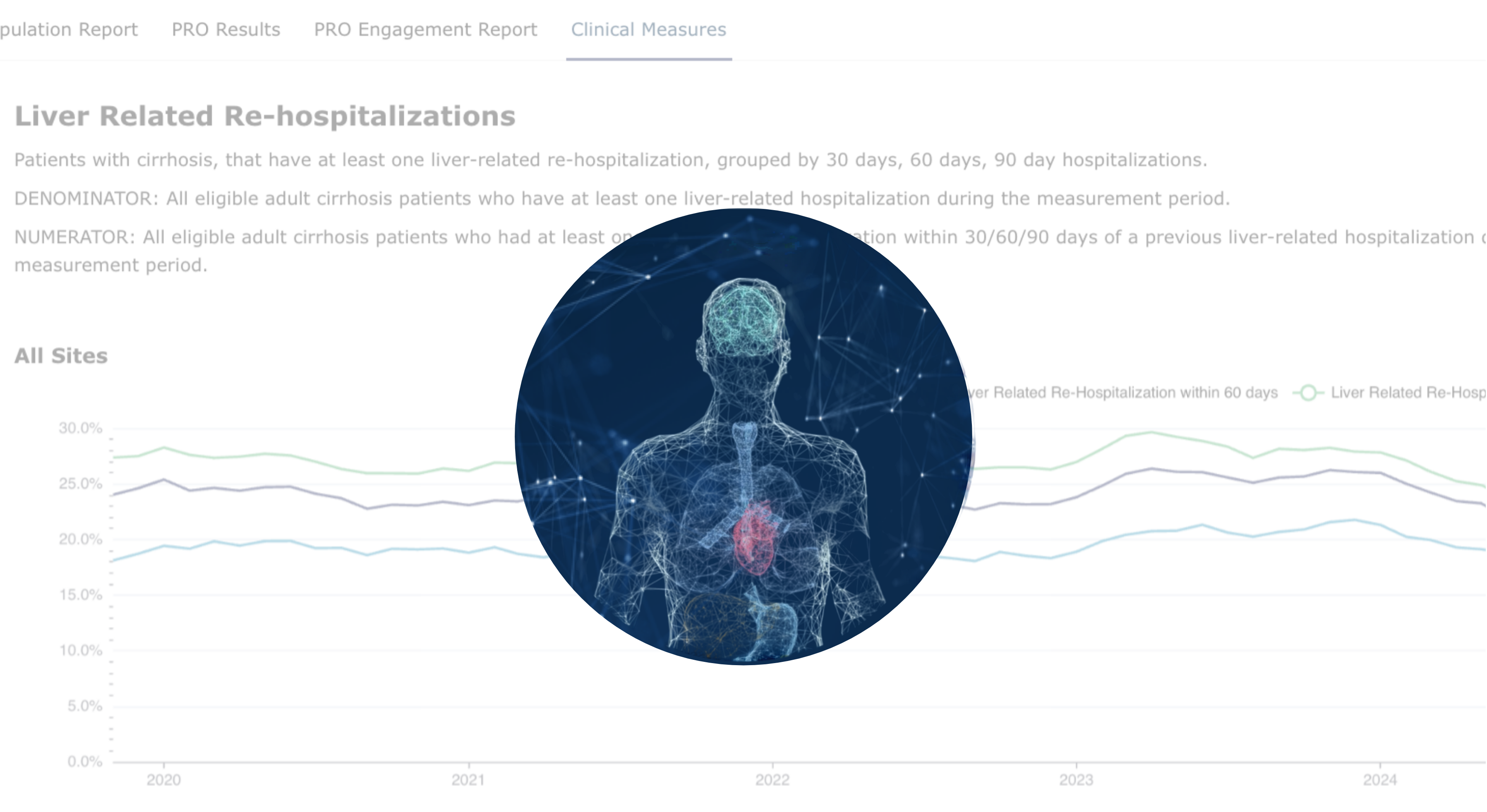
Disease Activity & Severity Scores
Track the progression of liver disease across the entire spectrum of chronic liver conditions, including Metabolic Associated Steatohepatitis (MASH), Primary Sclerosing Cholangitis (PSC), Cirrhosis, Primary Biliary Cholangitis (PBC), Alpha-1 Antitrypsin Deficiency, Wilson's Disease, Hepatitis C Virus (HCV), Hepatitis B Virus (HBV), and Hepatocellular Carcinoma (HCC).
Utilizing a combination of clinical data, laboratory tests, and imaging studies, we develop robust risk stratification algorithms and prognostic models. By incorporating data from liver biopsies, Fibroscans, and advanced fibrosis scores like APRI, FIB-4, ELF, BARD, SAFE Score, and NAFLD Fibrosis Score, Target RWE produces valuable insights into disease severity and progression. This comprehensive approach enables us to better understand the patient journey and identify potential therapeutic targets.
ALBI
APRI
MELD
Mayo Score
NAS
Globe Score
Child Pugh Score
NAFLD Fibrosis Score
ELF
BARD
SAFE Score
Milan Criteria
Proud Partners with the American Association for the Study of Liver Diseases (AASLD)

To accelerate the development of innovative therapies and improve patient outcomes in liver disease by leveraging real-world evidence and fostering collaboration between industry, academia, and clinical practice.
By collaborating with AASLD, Target RWE aims to leverage real-world data to improve patient outcomes and inform clinical practice. The registry’s extensive data supports the CQC initiative by enabling the identification of best practices and the development of evidence-based guidelines.
Learn MoreThe CQC partnership with AASLD has enrolled approximately 75,000 patients with various liver diseases, including MASLD, NASH, HBV, HCC, PBC, and PSC, enabling the study of disease progression and treatment outcomes.
Collaborating with Target RWE
Lead Liver Research
By joining, investigators and sites become part of the largest, most comprehensive, and technologically advanced hepatology research consortium.
Collaborate with Experts and Access Advanced Tools
This unique opportunity allows for collaboration with leading experts in the field and access to cutting-edge research tools and technologies.

Personalized Data Insights
Receive interactive quarterly reports and CQC dashboards tailored to your site's specific data.
Accelerated Research
Utilize individual site data for clinical trial cohort development, preliminary grant submissions, and feasibility analyses.
Concept Development
Develop robust research concepts and refine study designs.
Data Access and Analysis
Gain cloud-based access to relevant data and analytical tools (R and Python) to conduct in-depth analyses.

Discover Hidden Insights
Uncover trends, patterns, and potential research avenues within a vast dataset.
Drive Your Research Forward
Refine research questions, identify eligible patients, and collaborate with peers.